Introduction
SmartPulse Technology (SPT) is a new intelligent SCHWIND AMARIS feature perfecting corneal smoothness to optimise short-term clinical outcomes (up to two weeks after surgery). Long-term outcomes remain unchanged and are already documented through numerous existing AMARIS studies. The effectiveness of this new feature is presented here with the retrospective evaluation of the initial TransPRK outcomes internationally collected among eight SCHWIND reference clinics.
Surface treatments seem to be the most adequate test bed to appraise smoothing effects since the stromal surface is not initially assisted by the LASIK flap or the epithelium acting as smoothing agent. This is clinically supported by the fact that visual recovery typically takes longer than with stromal ablations. Moreover, TransPRK was selected, because it requires some 15,000 to 20,000 extra pulses to remove the epithelium, and this may potentially increase residual roughness.
Results
Visual Outcomes
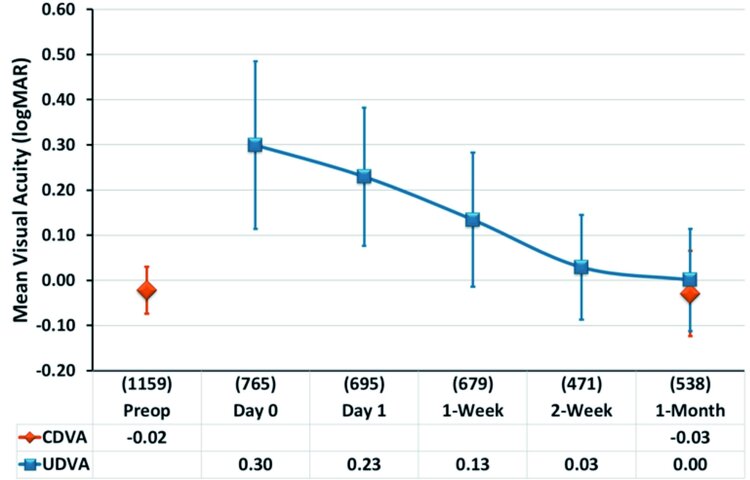
According to figure 1, the mean uncorrected distance visual acuity (UDVA) was 20/27 (0.13±0.15 logMAR) one week postoperatively. Among all contributing centres, UDVA ranged from 20/25 (0.10±0.14 logMAR) to 20/36 (0.26±0.16 logMAR). These results confirm excellent visual performance in the early postoperative stage compared to the reported visual recovery after sur-face treatments. In the words of the study participant David T. Lin, Canada: “We are at the front edge of a paradigm shift in refractive surgery. Most patients comment that shortly after surgery, they are seeing almost as well as previously with their glasses. SmartPulse has brought the ‘Wow’ effect to transepithelial PRK.”
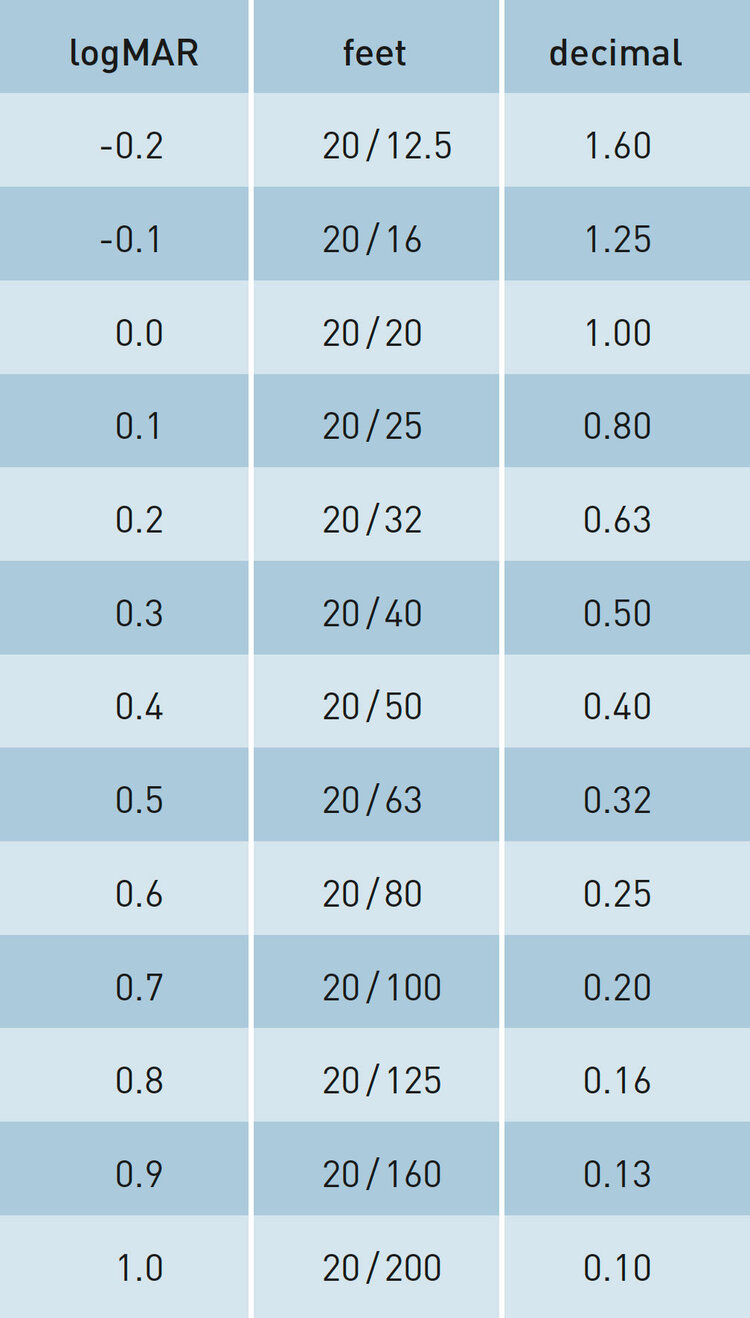
The one month UDVA of 20/20 (0.00±0.11 logMAR) already showed equal performance compared to the preoperative corrected distance visual acuity (CDVA). Among all contributing centres, UDVA ranged from 20/16 (-0.09±0.10 logMAR) to 20/25 (0.09±0.12 logMAR). The one month results are very similar to the outcomes obtained so far with AMARIS technology and confirm the high quality and stability of clinical results.
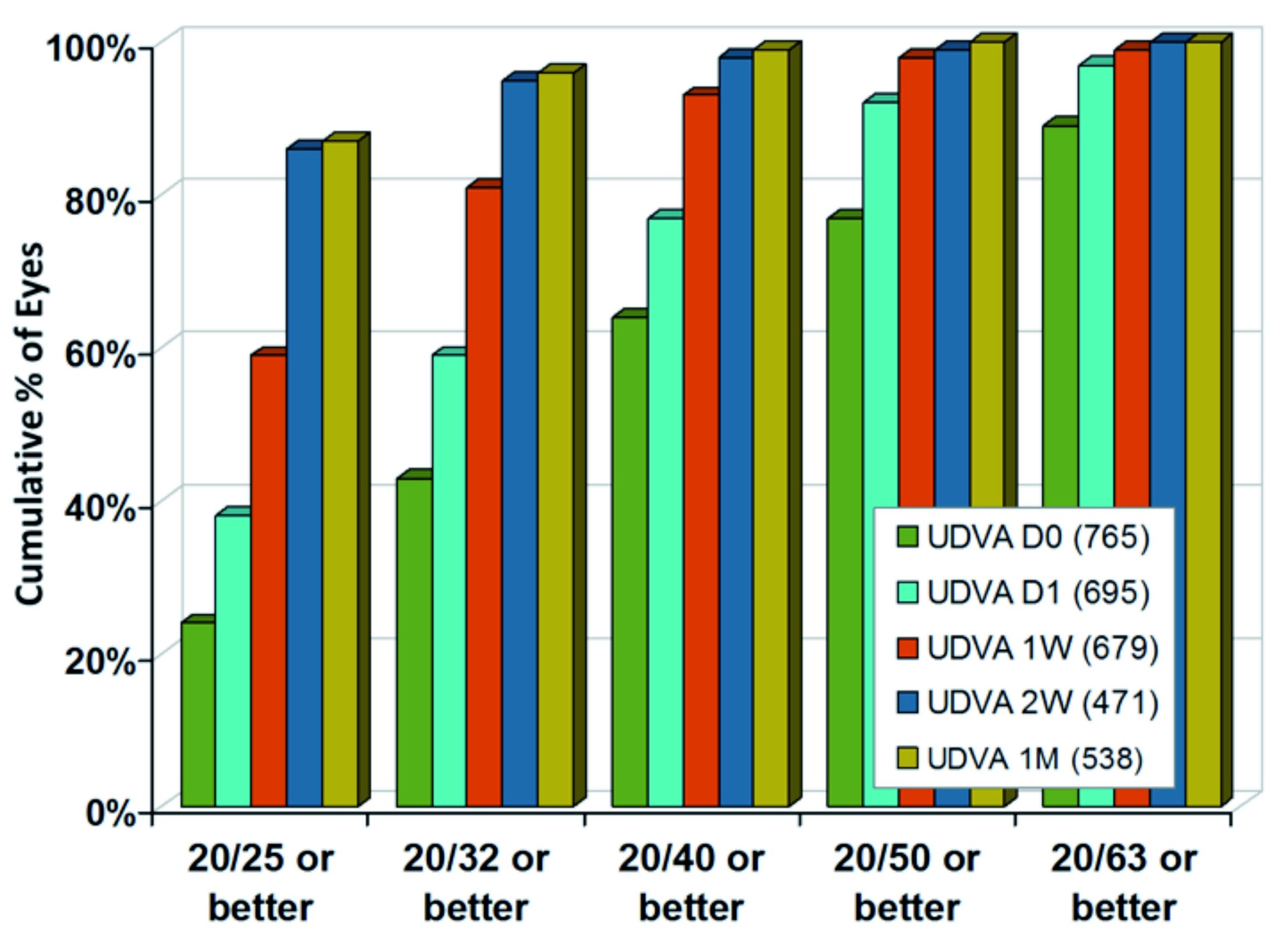
Figure 2 displays the cumulative distribution of visual outcomes ranging from immediate after ablation (D0) to one month results (1M).
Taking 80 % as a representative level for the population, 89 % of treated eyes reached 20/63 immediately after ablation, compared to 92 % reaching 20/50 on the next day, 81 % reaching 20/32 after one week and 86 % reaching 20/25 after two weeks, remaining stable to one month.
Among all contributing centres, between 52 % and 84 % of eyes achieved UDVA of 20/40 or better immediately after ablation.
In the words of another study participant, Michiel H.A. Luger, Netherlands: “Immediately at the end of the procedure it is striking how smooth the stromal bed is. This SmartPulse Technology is reflected in good vision on day one. Now, even TransPRK patients walk out the door with reassuringly good vision right after surgery.”
One day after ablation, 92 % of eyes achieved 20/50 or better (0.4 logMAR or better). Among all contributing centres, between 81 % and 100 % of eyes achieved UDVA of 20/50 or better.
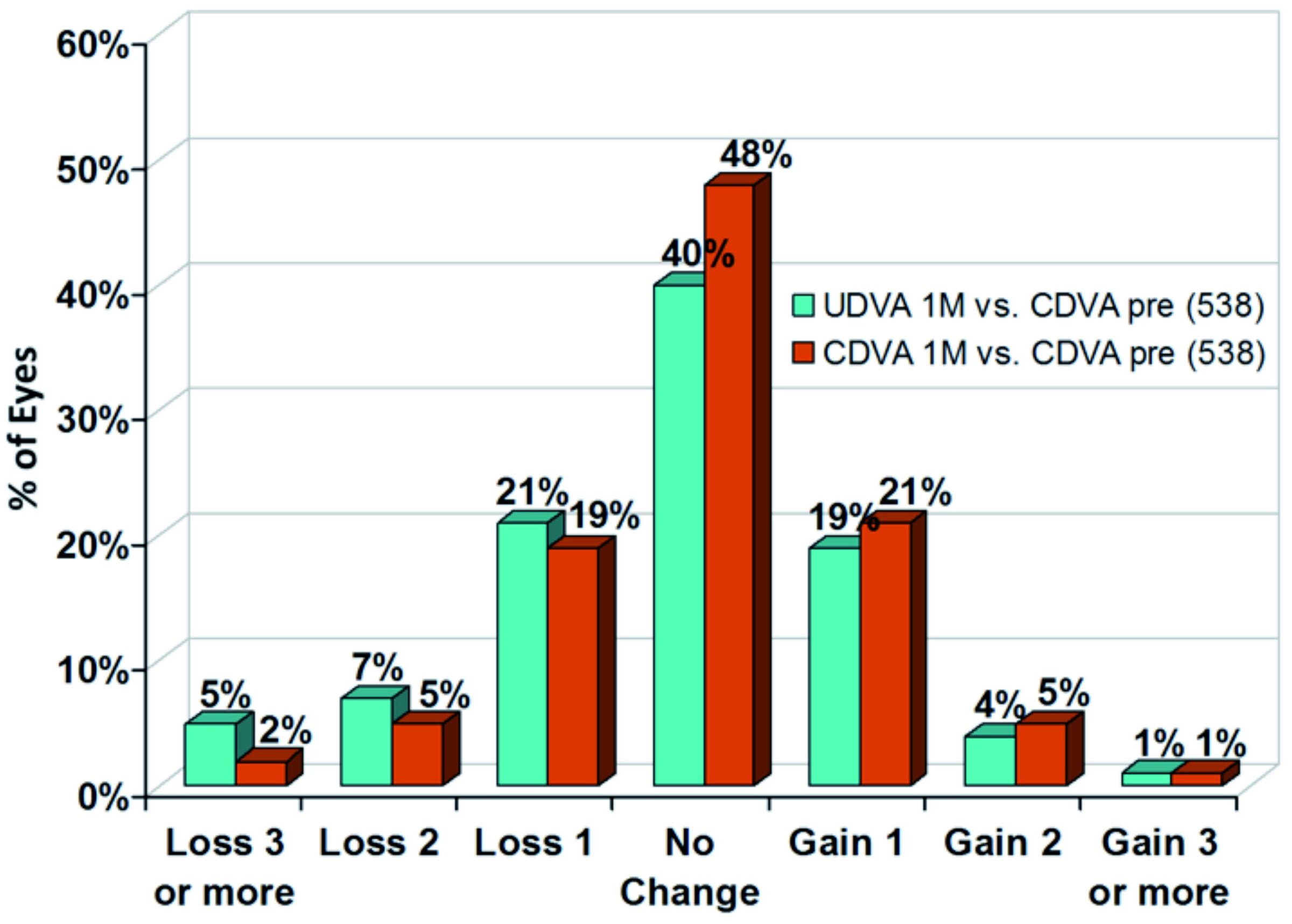
In figure 3, the review of 538 eyes using SPT documents high safety. At one month postoperatively, in 75 % of eyes no loss in Snellen lines (CDVA) was observed. 27 % of eyes even gained one or more Snellen lines.
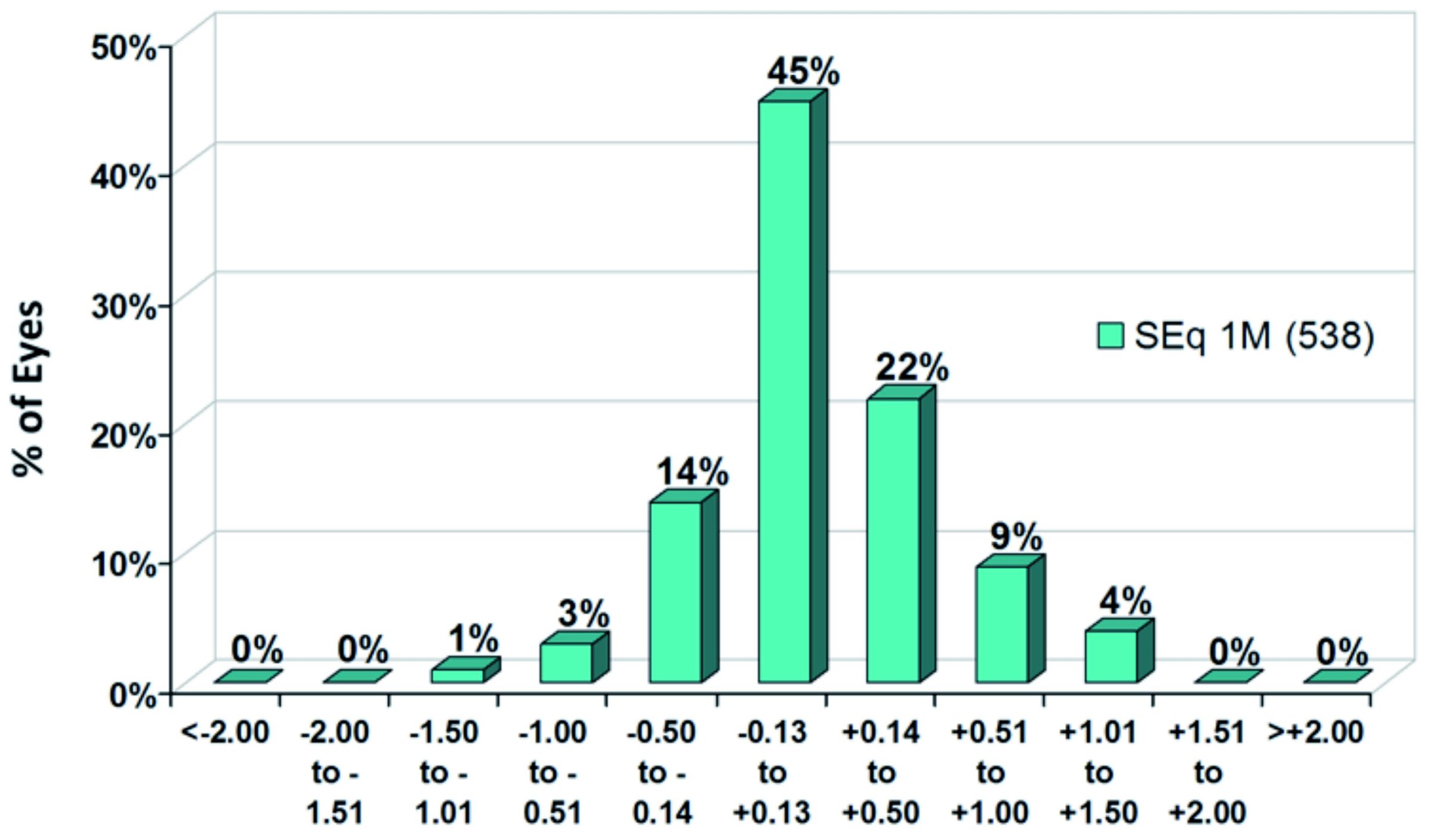
Figure 4 shows, that the predictability of the target refraction was also very good. At one month postoperatively, mean spherical equivalent (SEq) was +0.13±0.42 D. 81 % of 538 eyes were within the range of ±0.50 D from target SEq, 45 % achieved the target refraction even with a minimum deviation of only ± 0.13 D.
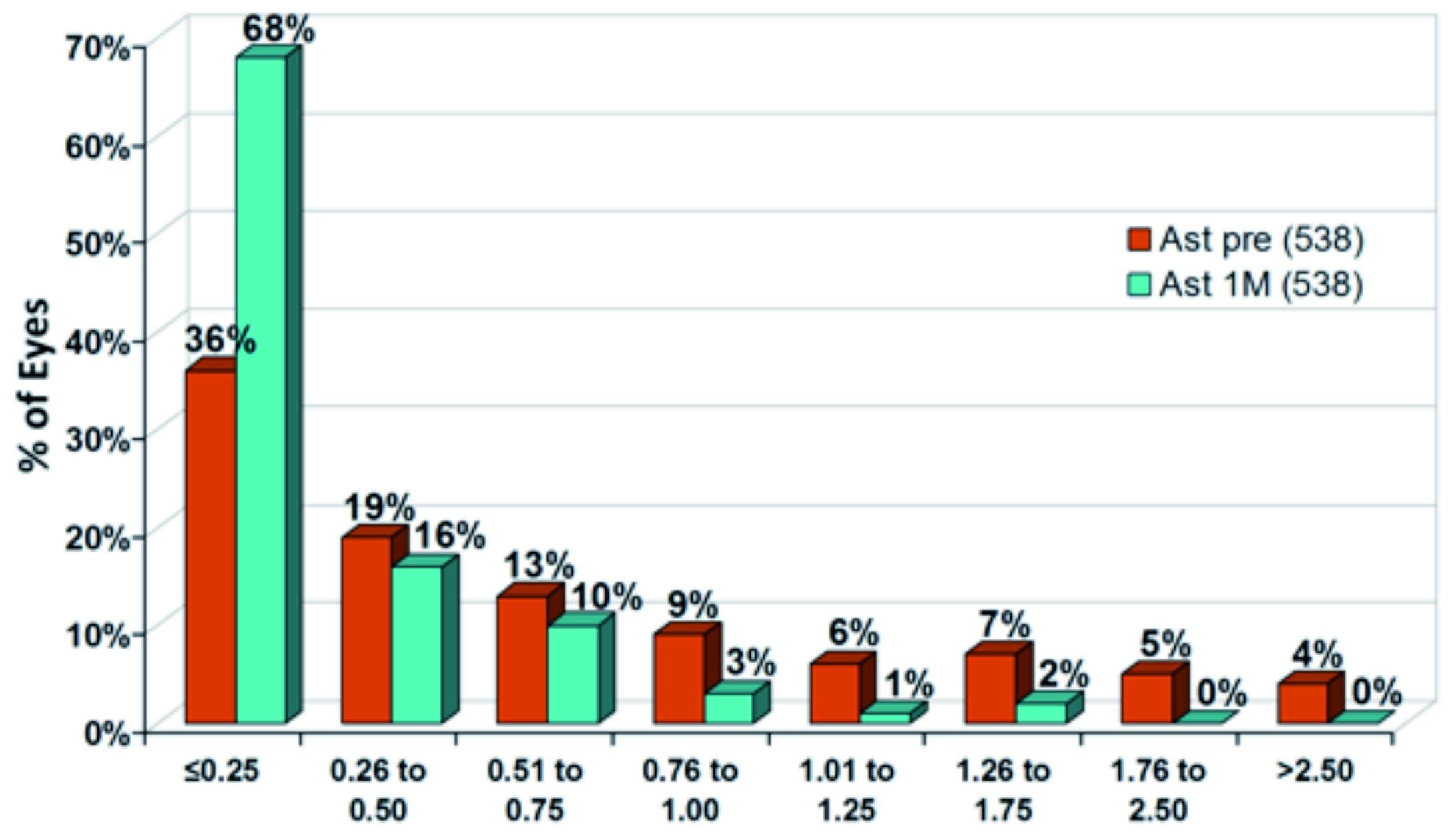
Figure 5 shows the cumulative distribution of astigmatism. At one month postoperatively, mean astigmatism was small with 0.3±0.3 D. 84 % of eyes had a residual astigmatism of 0.50 D or less, 68 % of eyes even had only 0.25 D or less.
Single Centre Comparison
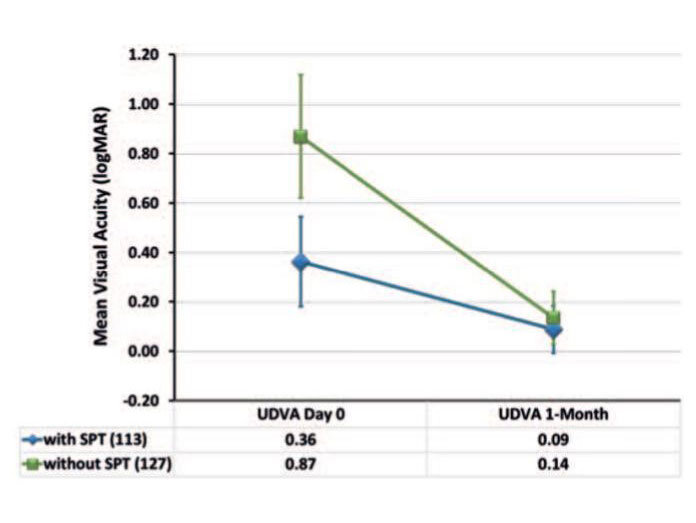
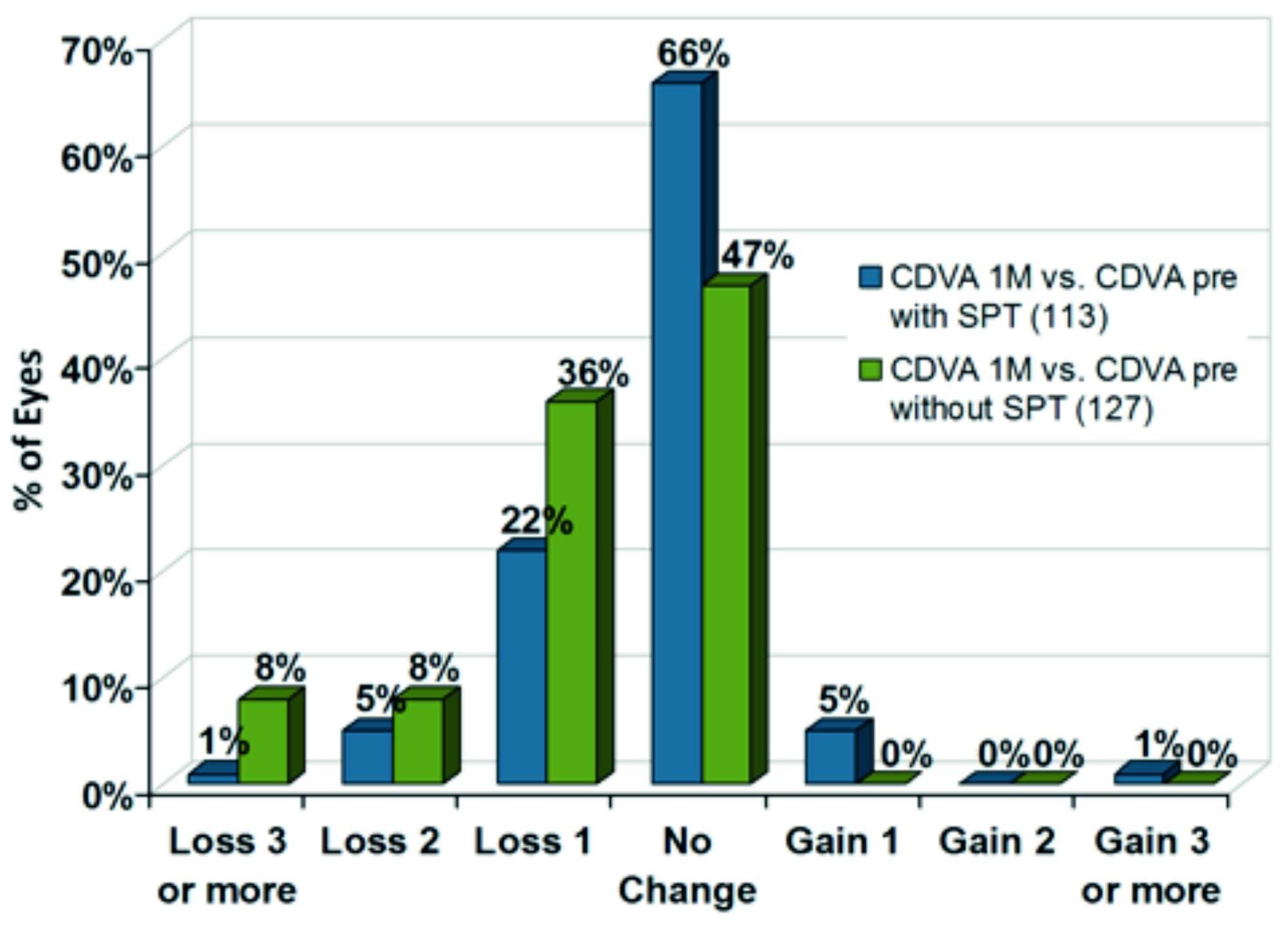
The evaluation of data from a single centre compared to a previous AMARIS cohort included comparison of 240 eyes treated with and without SPT (figures 6, 7, 8). The most significant difference between both approaches can be observed immediately after surgery with a mean difference of about five Snellen lines when using SPT. At one month postoperatively, the difference was less significant with a mean difference of about 0.5 Snellen lines better when using SPT (figure 6).
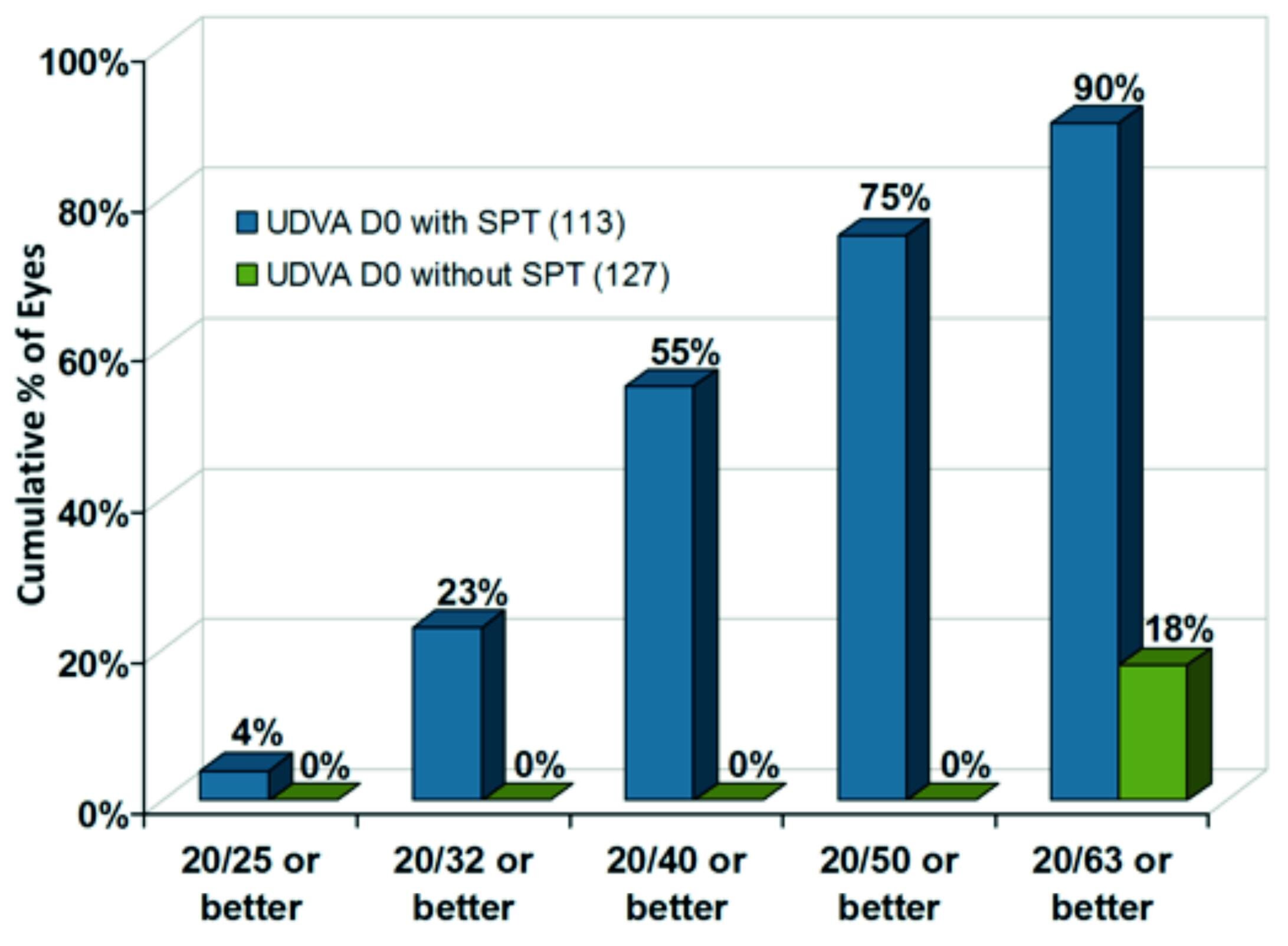
Immediately after surgery, an impressive increase of UDVA was observed. 90 % of eyes achieved 20/63 or better with SPT, whereas only 18% of eyes achieved this result without SPT. Even 23 % of eyes obtain an UDVA of 20/32 with the new approach, resulting in a difference of more than three Snellen lines better when using SPT (figure 7).
Using SPT, a high level of safety can be stated. At one month postoperatively, in 72 % of eyes no loss in Snellen lines was observed. 6% of eyes even gained one or more Snellen lines (figure 8).
Conclusion
SmartPulse provides excellent results, particularly in the early postoperative stage after surgery. The contributing surgeons reported shorter recovery time of visual acuity, higher levels of postoperative visual quality, and shorter re-epithelialization time using SPT. Moreover, most of their patients commented that, immediately after surgery, they were seeing almost as well as previously with their glasses.
The early outcomes are even further remarkable in that they were ob-tained with surface treatment (in particular TransPRK) where a longer visual recovery is commonly observed in the early postoperative stage (as opposed to LASIK).
At one month postoperatively, refractive outcomes with or without SPT are very similar and confirm the stability and quality of outcomes already achieved with AMARIS prior to SPT.
In summary, SPT meets the require-ments set for it. SPT enhances short-term outcomes by reducing residual roughness and improves smoothness of the residual bed without compromising stability of long-term outcomes.